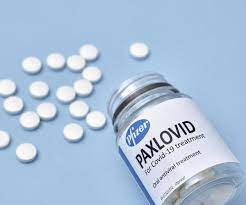
Earlier this week, President Biden outlined new steps to confront the growing spread of Covid-19 from the new, more infectious Omicron variant, which, in only a few weeks, has soared from virtually nonexistent to 73 percent of all new cases. Unfortunately, Biden’s plan failed to include what could be the most important action of all: an all-out effort to make safe and effective anti-viral Covid-19 pills available—two of which have now received Emergency Use Authorization from the FDA—to every American at the earliest sign of infection.

In less than two years, Covid-19 has caused more than 800,000 deaths and been diagnosed in 51 million Americans. The pandemic’s toll in suffering, health-care costs, and loss of productivity are huge and continuing to accrue. Omicron, the newly dominant variant, is so transmissible that Covid cases are surging not only in unvaccinated people but also as “breakthrough” infections in the vaccinated.
We have essentially three ways to manage a pandemic: testing and physical and behavioral controls to limit spread, including isolation, masking, quarantine, and tracking and tracing; vaccination to foster individual immunity to the infecting virus; and individual treatment regimens that reduce transmission and the severity of the illness. The U.S. has struggled to achieve an optimal, consistent level of testing, vaccination, and physical and behavioral controls.
Vaccines developed under then-President Trump in record time and made available by him and President Biden have been invaluable for protection from infections, serious illness, and death. However, only around 60 percent of Americans have been fully vaccinated, with about 30 percent having received an additional booster dose. The effectiveness of vaccination is limited by the characteristics of the coronavirus variants, which can change the duration and strength of recipients’ immune response to the vaccines. For example, vaccination appears to provide significantly less protection against Omicron infection in the absence of a booster. Treatment options have been limited because, until now, they have included only injectable products such as the anti-viral drug remdesivir and monoclonal antibodies, which have helped reduce hospitalization duration and mortality but are mostly ineffective against the Omicron variant.
Now, that is changing, with the recent appearance of FDA-authorized oral home-use medications. The most important of these is Pfizer’s Paxlovid, a combination of two drugs, nirmatrelvir and ritonavir, which was authorized by the FDA for emergency use on December 22. Nirmatrelvir is the first new drug designed specifically for use against coronaviruses, and ritonavir is an HIV drug in a very low dose to boost nirmatrelvir. The combination achieved an 89 percent reduction in hospitalizations of high-risk patients when taken within three days of the onset of symptoms and reduced tenfold the ability of the virus to replicate. Paxlovid shortens the period of contagiousness for people who become infected, a particular benefit for health-care and other frontline workers. (Of lesser significance is Merck and Ridgeback Biotherapeutics’ antiviral pill, which received Emergency Use Authorization from the FDA on December 23. Its efficacy in preventing hospitalization is about 30 percent.)
Related Content: Biden’s General Are Fighting the Last COVID War
But regulatory authorizations are little more than symbolic if the drugs are unavailable. If all Americans had rapid access to Paxlovid, early intervention would not only effectively treat those infected but would also arrest the spread of the Covid-19 virus and flatten the curve of infections. Of course, people would also need to have testing available to confirm that an illness was Covid-19 rather than the flu or some other disease.
Our optimism about Paxlovid is tempered by the White House’s failure to mount the aggressive initiative necessary to make it widely available. White House Coronavirus Coordinator Jeffrey Zients said on The News Hour that there would be 10 million courses of Paxlovid available by “late summer” of next year—which is too little, too late. The daily case count in the U.S. is around 200,000 and rising.
So that every American family can have timely access to Paxlovid treatment, the president should invoke the Defense Production Act (DPA), which grants him broad authority to direct private companies to help the government in emergencies. Using it, he could mobilize Pfizer, Merck, and other companies to make as many pills as possible, even at the expense of less critical products. As Eric Topol, director of Scripps Research Translational Institute, emphasizes about the pills, “This is a remedy. It doesn’t depend on our immune response”—and thus should be acceptable to people who, for one reason or another, have shunned vaccination and avoided masking.
The White House should immediately act on these potentially transformational drug treatments. Failing to do so will unnecessarily prolong and increase the health and economic costs of the pandemic.
![]()
John J. Cohrssen is an attorney who has served in many government posts in both the executive and legislative branches of government, including as counsel for the House Energy and Commerce Committee. Henry I. Miller, a physician and molecular biologist, is a senior fellow at the Pacific Research Institute. He was the founding director of the FDA’s Office of Biotechnology.

